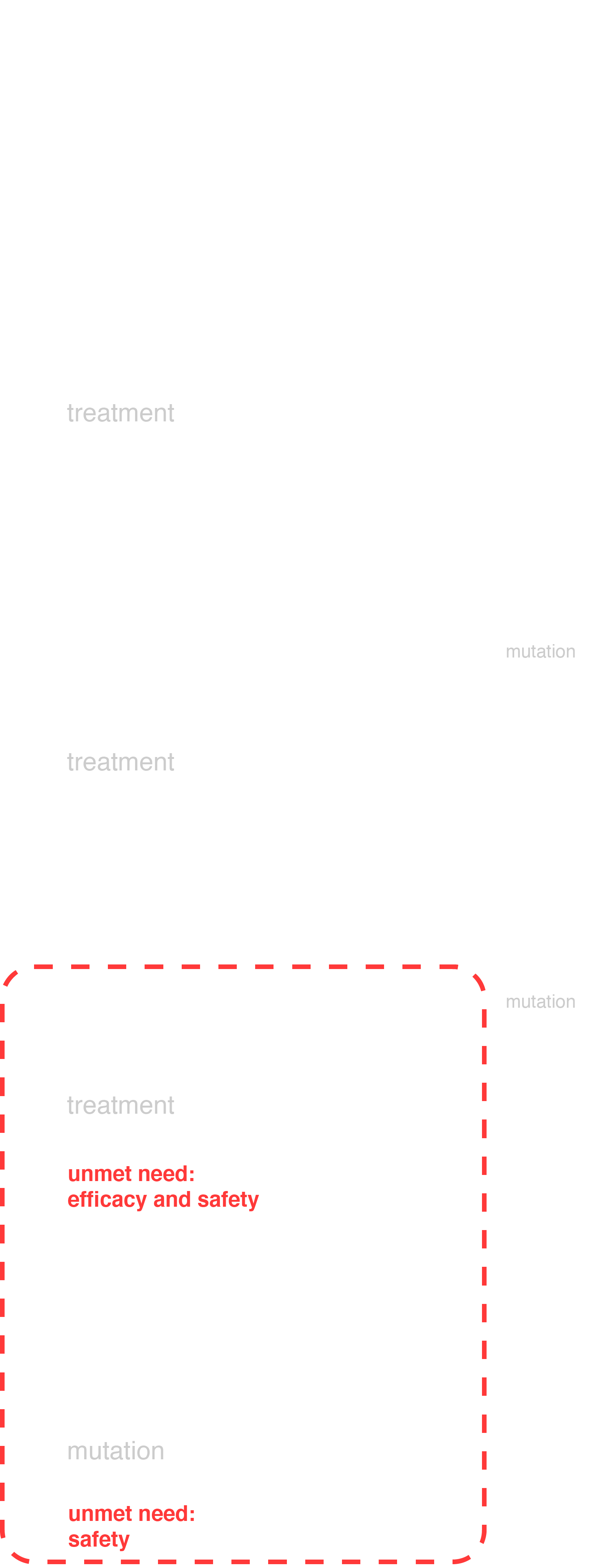
Unmet need
At present, inhibitors of tyrosine kinase Abl are used for the therapy of CML, with already 3 such inhibitors (imatinib, dasatinib and nilotinib) approved for the therapy of primary patients and one more — bosutinib — has a high chance of being approved. Nevertheless, despite the high effectiveness of drugs in the first line of therapy, a small proportion of patients may acquire resistance or intolerance, which requires switching to another drug. Due to the high survival rate of patients with CML (median more than 10 years), the number of such events increases with time, and patients can switch to the 3rd and later lines of therapy. The effectiveness of drugs in this line of therapy is already significantly lower, which is an important medical problem. Also, patients can acquire a T315I mutation in the therapy of which currently only the preparation of the third generation of ponatinib is effective, which, however, has serious side effects (thrombotic and cardiovascular events, etc.).
Thus, CML therapy in the 3rd line and in patients with T315I mutation requires a more effective and safer drug in addition to those already available. To meet this need, a new selective inhibitor of the 3rd generation Abl—PF-114 is targeted.
Thus, CML therapy in the 3rd line and in patients with T315I mutation requires a more effective and safer drug in addition to those already available. To meet this need, a new selective inhibitor of the 3rd generation Abl—PF-114 is targeted.

Unmet need
At present, inhibitors of tyrosine kinase Abl are used for the therapy of CML, with already 3 such inhibitors (imatinib, dasatinib and nilotinib) approved for the therapy of primary patients and one more — bosutinib — has a high chance of being approved. Nevertheless, despite the high effectiveness of drugs in the first line of therapy, a small proportion of patients may acquire resistance or intolerance, which requires switching to another drug. Due to the high survival rate of patients with CML (median more than 10 years), the number of such events increases with time, and patients can switch to the 3rd and later lines of therapy. The effectiveness of drugs in this line of therapy is already significantly lower, which is an important medical problem. Also, patients can acquire a T315I mutation in the therapy of which currently only the preparation of the third generation of ponatinib is effective, which, however, has serious side effects (thrombotic and cardiovascular events, etc.). Thus, CML therapy in the 3rd line and in patients with T315I mutation requires a more effective and safer drug in addition to those already available.
To meet this need, a new selective inhibitor of the 3rd generation Abl—PF-114 is targeted.
Our solution
PF-114 is a 3-rd generation small-molecule Abl inhibitor, which, unlike ponatinib, has fewer side kinase targets, which allows for greater safety of PF-114 while maintaining efficacy, including the mutant form of T315I.
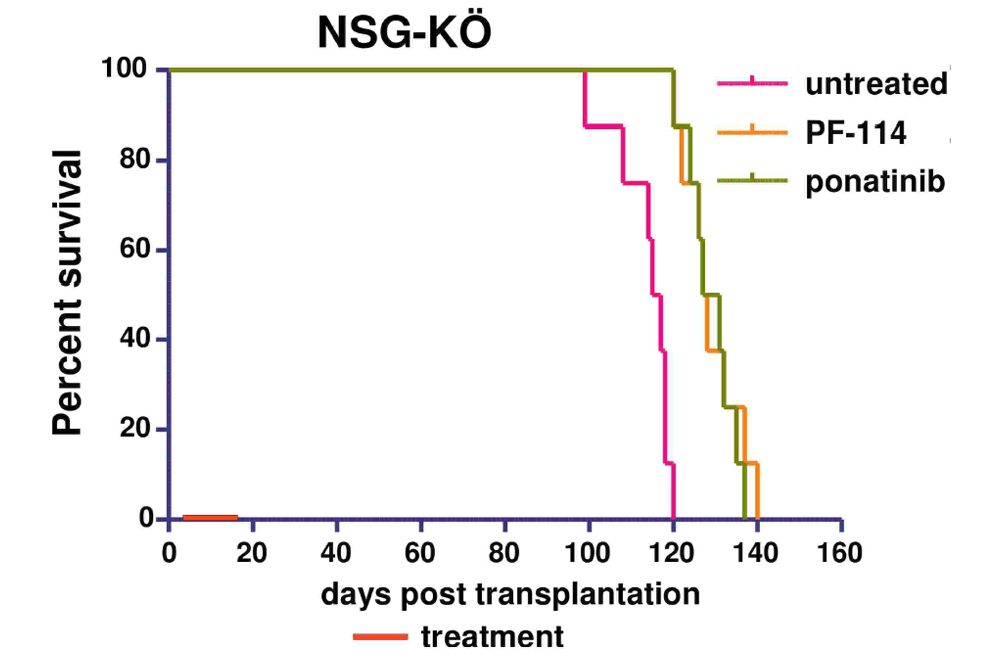
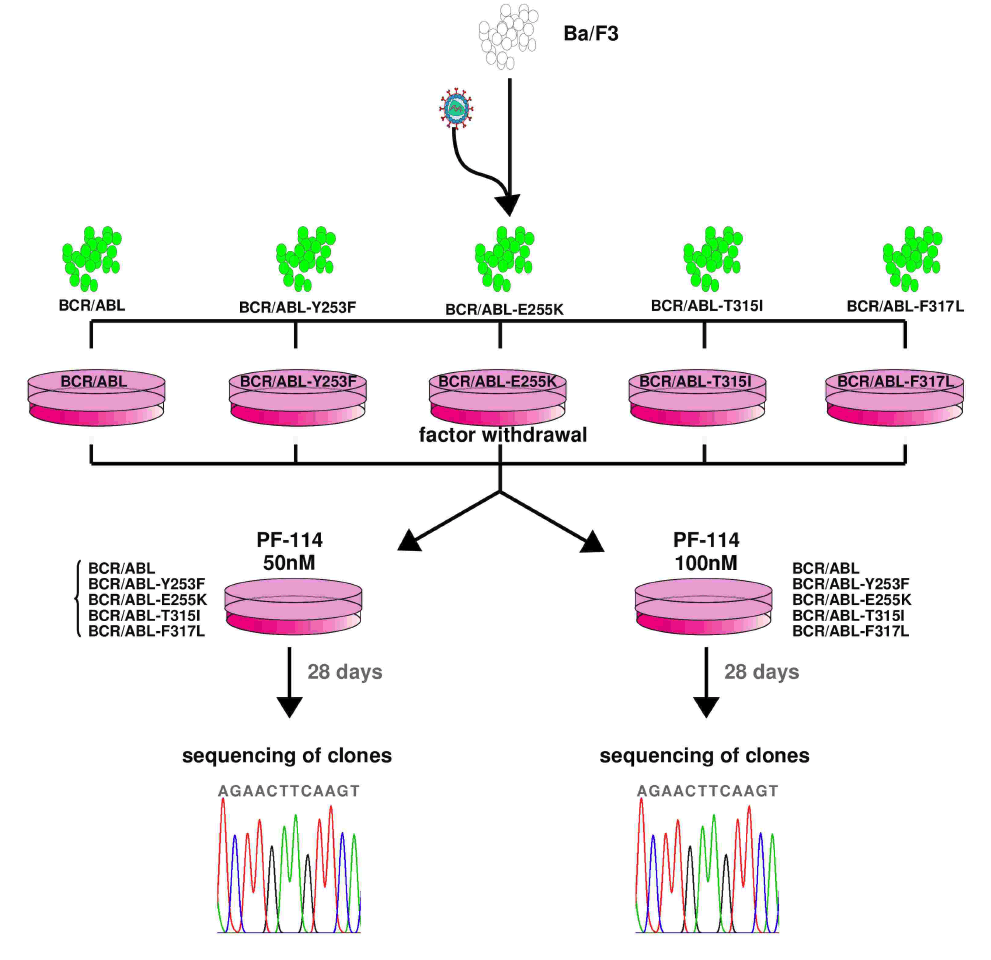
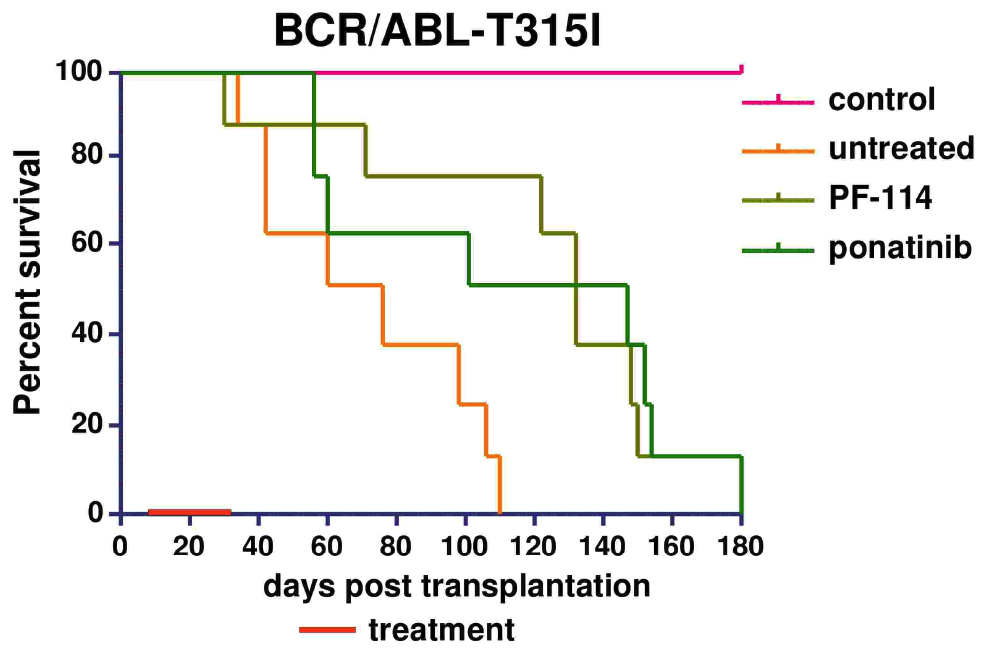
In numerous preclinical experiments, the efficacy of PF-114 was shown against Abl and various mutant forms including T315I, suppression of emergence of new mutations in in vitro systems, and was effective in CML animal models and Ph + ALL with native Abl form and T315I mutation.
Pipeline
Clinical development of PF-114 includes the first phase of clinic al trials (ongoing), which examines the efficacy, safety, pharmacokinetics of different doses of the drug in patients with resistant forms of CML, including the mutation T315I. In the future, a multicentre in ternational clinical study of the 2nd phase is planned according to the indication of CML in the 3rd line of therapy and with the T315I mutation. In addition, Phase 2 studies can be performed on the indications of CML i n advanced phases (acceleration and blast crisis) as well as Ph + acute lymphoblastic leukemia.


Publications
Mian AA, Rafiei A, Haberbosch I et. al. PF-114, a potent and selective inhibitor of native and mutated BCR/ABL is active against Philadelphia chromosome-positive (Ph+) leukemias harboring the T315I mutation. Leukemia. 2015 May;29(5):1104−14
Turkina AG, Vinogradova OYu, Lomaia EG et. al. Phase-1 Study of PF-114 Mesylate in CML Failing Prior Tyrosine Kinase-Inhibitor Therapy, 60th ASH annual meeting, Dec 1-4, 2018
Turkina AG, Vinogradova OYu, Lomaia EG et. al. Phase-1 Study of PF-114 Mesylate in CML Failing Prior Tyrosine Kinase-Inhibitor Therapy, 60th ASH annual meeting, Dec 1-4, 2018
Intellectual property
Fusion Pharma conducts an active international protection of intellectual property rights. National patents have been received for PCT/RU2012/000423 with a priority date of 2012. In 2017, a PCT application PCT/RU2017/050025 was filed for the salt form of the drug with a priority date of 2016 and patents in some of the countries already granted. In 2018, additional PCT application PCT/RU2018/050131 was filed for the dosage and formulation of the drug with priority date of 2017.


